Company Information
History
Incorporated in 1994 as Posh Chemicals Private Limited, it has is as Valens Molecules Private Limited in 2016. The etymology of VALENS originates from 'Valency' - meaning - the combining capacity of an atom. VALENS also means strong, vigorous, capacity and healthy. VALENS truly represents our vision to be stronger, healthier and bigger and compliments our goal to nourish strong bond with our customers and vendors.
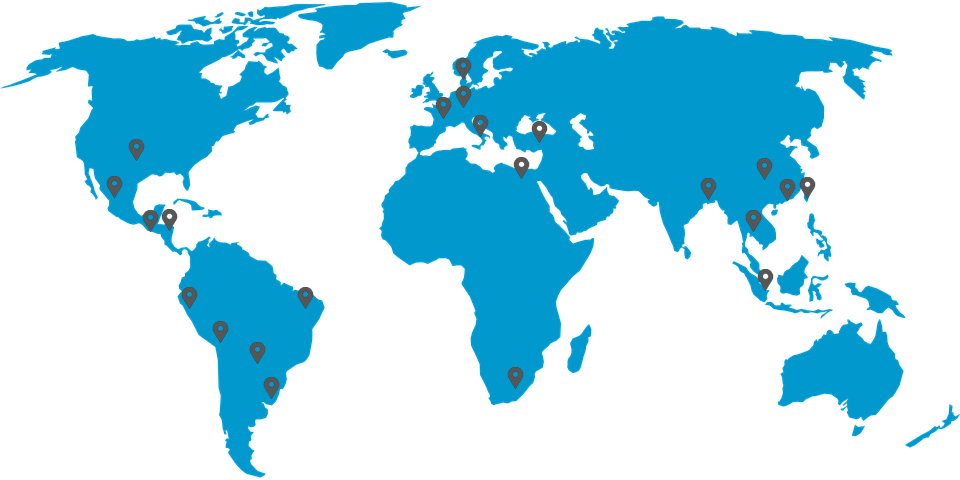
Starting our first export business 1998 we are now serving customers across 5 continents spreading over 30 countries. The company is US FDA inspected since 2002 and received the EU GMP in 2014.
Quality Policy
Environmental Policy
Environments are not just containers, but are processes that change the content totally
-Marshall McLuhan
At Valens Molecules, everyday is an earth day. The emissions, effluents and wastes from our facilities are an important environmental consideration. To minimize our environmental footprint, we design processes that reduce our demand for hazardous materials, minimize the generation of waste, and establish systems to reuse or recycle materials. Posh is committed to continually improve its operations to prevent pollution of the environment, handling of hazardous waste and control of toxic emissions. Conservation of natural resources and utilities by complying with statutory environmental requirements is one of our strongest ethical practices.
Global Presence
Certifications
-

USFDA
-

WHO GMP
-

Mexican GMPP
-

EDQM GMP
-

ISO certificated
-

EDQM
